Here's a normal comment from my chemistry course...
"The equation
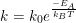
seems to suggest that the rate constant, 'k', is dimensionless since activation energy over the boltzmann constant times temperature is joules over joules in dimension. However, this problem and the book indicate that it's in inverse seconds. And all of this is confusing me in my calculations. Could someone clarify this for me? -
saturnianalien
("All this is confusing me" -You're not alone!)
Someone responds...
"The dimension of k is 1/s. the exponential function is dimensionless (both EA and kB*T have the dimension of energies, but k0 has the dimension 1/s."
Also, zeroth is a word (It's in my textbook)
No comments:
Post a Comment
Please comment, I like feedback!